Table of Contents
Change Of State
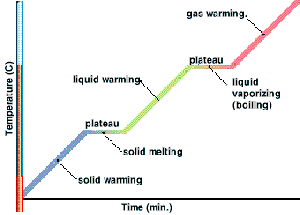
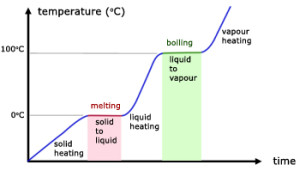
When a pure solid is heated, its temperature rises until it starts to melt. At its melting point, any additional heat supplied will not change its temperature. When the pure solid becomes a pure liquid (a change in state), further heating will again raise the temperature of the liquid until it starts to boil.
At its boiling point, any additional heat supplied causes boiling without any temperature rise. When the pure liquid becomes a pure gas (a change in state), further heating will again raise the temperature of the gas.
Therefore, at particular temperatures, heating changes the state of the substance. Melting and boiling are such processes. Similarly, at almost the same particular temperatures for the same substance, cooling can also change its states. Condensing and freezing are such processes.
The properties of the molecules of the substances vary with the amount of thermal energy they possess.
Important: During the changing of state, the temperature of the gas/liquid/solid is constant.
Melting
Melting is the change of state from a solid to a liquid. Melting of a pure substance occurs at a particular constant temperature called melting point.
- The molecules in a solid, which are bound by inter-molecular forces, vibrate around their equilibrium position in a crystalline structure. During melting, the molecules gain energy to weaken the intermolecular attractive forces and increase the distance between the molecules (increasing their potential energy while keeping kinetic energy constant), thus acquiring the greater degree of freedom and disorder.
The change of state from a liquid to a solid is called solidification or freezing or casting. A pure substance freezes at a temperature equal to its melting point.
In most of the substances, melting causes expansion and freezing causes contraction. Water is an exception. Ice melts to water causes contraction and water freezes to ice causes expansion. Density of water is highest at 4°C – water reaches minimum volume. This is why ice floats on water.
From the graph above,
- $0^{\circ}\text{C}$ is the melting point of the solid (which is ice in this case).
- Temperature remains constant at $0^{\circ}\text{C}$ as the solid is melting (Why? Answer is below).
- All energy supplied is “directed” to “melting” the solid.
- During the melting process, solid and liquid exist in equilibrium.
Step-By-Step Process Of What Happens During Melting
- Heat energy is absorbed by the particles
- Heat energy is converted to kinetic energy
- The kinetic energy of the particles increases and the particles in the solid vibrate faster
- At melting point, the particles have gained enough energy to overcome the attractive forces between particles
- Particles starts to move away from their fixed position
- Liquid is formed
Cause For Constant Temperature During Melting
The absorbed heat energy during melting is used to weaken the attractive forces between particles and not the kinetic energy of the particles.
Factors That Affect Melting Point
Melting point is affected by purity of sample and pressure on the sample.
When impurities are mixed with a pure substance, the melting point is affected. This change in the melting point has its usefulness:
- In cold countries, water in pipe lines tend to freeze in winter season, where the ambient temperature drops to below $0^{\circ}\text{C}$. Freezing causes the water in the pipe to expand (recall that water expands when it freezes) and this might cause the pipe to burst. The common method to prevent this is to add antifreeze. With the addition of antifreeze, the melting point of the water + antifreeze mixture will drop to below $0^{\circ}\text{C}$ and hopefully, below the ambient temperature.
- Adding salt to water can reduce its melting point to as low as -18 °C. Salt is put onto the roads in cold countries during the winter season.
Mostly substance increases their melting point when a pressure is applied in their solid state.
- Normal solids such as iron, copper undergoes expansion when they melt. When pressure is applied on the surface of a normal solid, the expansion is suppressed and melting is delayed. Thus, the melting point of a normal solid is raised by the application of pressure.
- Abnormal solids, like ice and bismuth, contract on melting into liquids. When pressure is applied on the surface of such a solid, the change into the liquid is assisted by the increase in pressure. Thus, the melting point of ice is lowered by the application of pressure.
Even though the examples above are metals, the change in melting point due to application of pressure occurs for non-metals as well. An example will be ice. With the addition of pressure, the melting point of ice will be lowered.
Note: Freezing point of pure water is 0 °C at standard atmospheric pressure. (Melting point of a substance must be stated together with its purity and surrounding pressure.)
Worked Examples
Example 1
What makes ice effective for chilling beverages?
Click here to show/hide answer
Ice is particularly effective for chilling beverages because of its high latent heat of fusion. The high latent heat of fusion refers to the amount of heat energy required to change a substance from a solid to a liquid without a change in temperature. In the case of ice, it takes a substantial amount of energy to break the intermolecular bonds and transition it into water.
When ice is added to a drink, it absorbs heat from the warmer beverage and its surroundings. The high latent heat of fusion allows ice to absorb a significant amount of heat energy during the phase transition from solid to liquid (melting) without a noticeable increase in temperature. This energy absorption contributes to the cooling effect in the drink, helping maintain a refreshing and chilled temperature. The combination of ice’s ability to absorb heat and its high latent heat of fusion makes it a highly efficient coolant for beverages.