Table of Contents
Brownian Motion
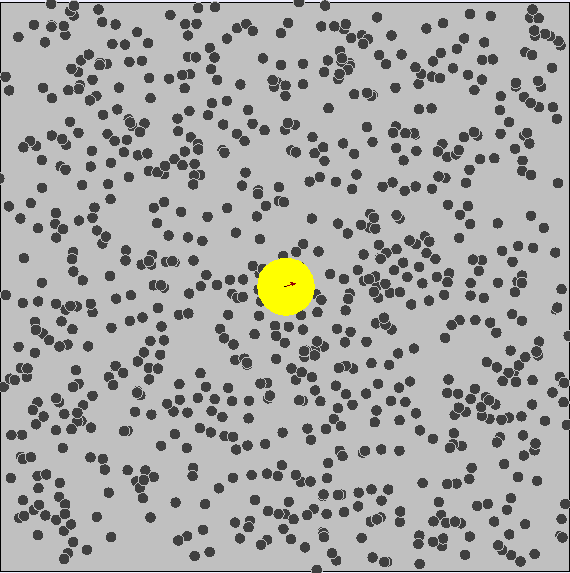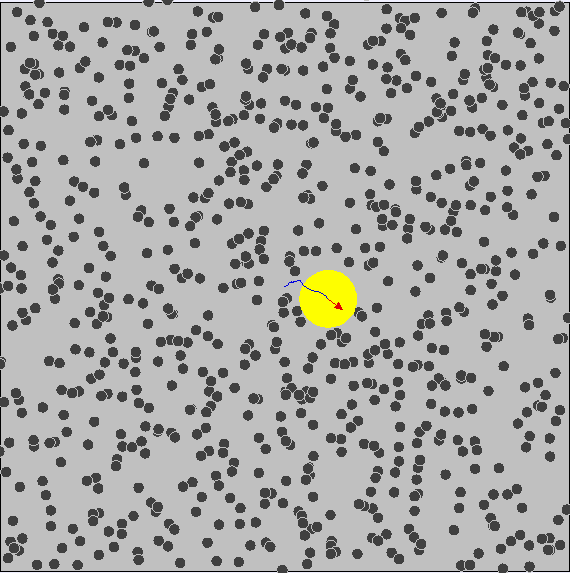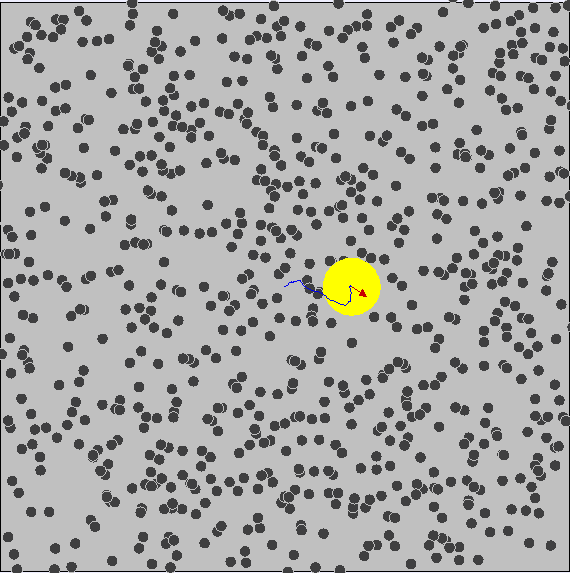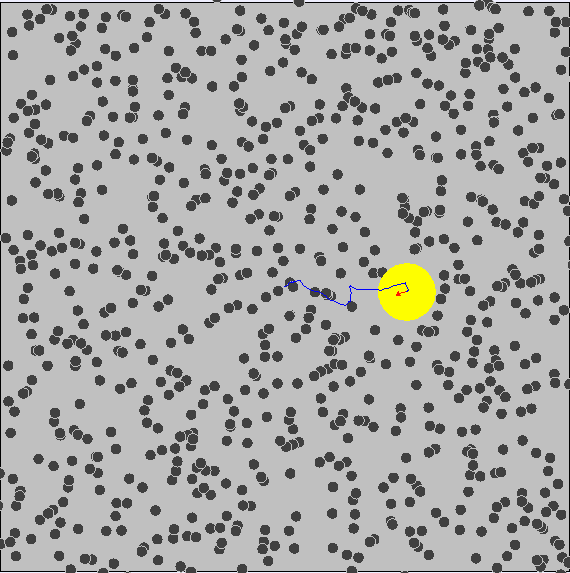
Brownian motion is the random and irregular motion of gas and liquid molecules. Brownian motion provides clear evidence for the kinetic molecular model of matter in that matter is comprised of tiny particles that are in continuous random motion, with a range of speeds n all directions and kinetic energies.
In the simulation above, it is seen that the particles are seen moving about in a random manner.
Why Does The Particles Move In A Random Manner?
- The observed irregular motion of the particles is due to the bombardment of air molecules. The air molecules are too small to be seen. The particles are continually bombarded unevely on different sides by air molecules. This results in the irregular movement of the smoke particles.
- The random motion of the particles demonstrates that air molecules move randomly in all directions with a range of speeds and kinetic energies.
As temperature increase, the brownian motion of the particles is more frantic. They will move about more vigorously.
Further Discussion On Brownian Motion
Brownian Motion is invented by botanist Robert Brown in 1827, Brownian motion refers to the random motion of particles suspended in a fluid (liquid or gas) resulting from their collision with the fast-moving atoms or molecules in the gas or liquid. It’s a phenomenon that governs our lives in ways more than one can imagine.
What Is Brownian Motion?
Firstly, let’s get to understand what exactly Brownian Motion is. It is a phenomenon where minute particles immersed in a fluid move in an erratic, random manner due to continuous bombardment by the surrounding fluid molecules. Robert Brown, a Scottish botanist first observed this under his microscope while studying pollen particles suspended in water. He noticed the particles were jittering around as if alive!
The intriguing part here is that these movements are purely physical, not biological. Therefore, Brownian motion is a beautiful manifestation of how molecular movement translates into visible macroscopic effect. It stands at the intersection between macroscopic and microscopic worlds, being instrumental in establishing the kinetic theory of gases and validating Einstein’s theory of relativity.
Brown’s discovery paved the way for future scientific theories and inventions. The explanation for this phenomenon, however, did not arrive until nearly 80 years later when Albert Einstein published a paper on it in 1905. Einstein mathematically analysed Brownian motion and provided a concrete proof of atomic theory.
The concept of Brownian motion has found its applications across various fields like physics, chemistry, mathematics and even in financial modeling! In physics, it forms the basis for the diffusion process and helps in understanding the thermal properties of substances.
How Does Brownian Motion Work?
You must be wondering how these tiny particles move so randomly. The underlying mechanism of Brownian motion lies in the kinetic theory of matter. All matter is composed of atoms and molecules that are continuously in motion. When a particle is suspended in a fluid, the fluid’s molecules, which are also in constant motion, collide with the particle.
These repeated collisions exert forces on the suspended particle from all directions. However, because the collisions are random and asymmetrical, the particle does not move uniformly, but instead zig-zags erratically. This movement is what we know as Brownian motion.
Interestingly, the velocity of Brownian motion depends upon the temperature and viscosity of the fluid in which the particle is suspended. An increase in temperature would speed up the fluid molecules’ motion, leading to more frequent and harder impacts, resulting in faster Brownian motion. On the other hand, a more viscous fluid would slow down both the fluid molecules and the suspended particles’ motion.
Lastly, Brownian motion continues as long as the particle remains suspended in the fluid and the fluid temperature remains above absolute zero. If the fluid were to freeze or if its temperature were to fall to absolute zero, both the fluid’s molecules and the suspended particles would stop moving, ending the Brownian motion.
The Impact Of Brownian Motion On Our Daily Lives
Though it might seem like an obscure scientific theory, Brownian motion has significant implications in our daily lives. It plays a crucial role in various processes like formation of aerosols in the atmosphere, operation of ink-jet printers and even in determining stock market prices!
In medical science, Brownian motion is instrumental in understanding diffusion process within human body. In technological aspects like nanotechnology, scientists manipulate Brownian motion to position tiny objects. In environmental science, it explains how pollutants spread in the air or water.
The concept is also used in mathematical modeling and prediction algorithms. For instance, in finance, mathematicians use Brownian motion to predict the random movement of stock market prices. These mathematical models using Brownian motion are collectively known as Stochastic processes, used widely in various fields like finance, economics, and even physics.
Conclusion
In essence, Brownian motion is a captivating dance of particles, an unseen ballet that unfolds at the most microscopic level yet has an impact on a macroscopic scale. It is not only fundamental to our understanding of physics but has a significant influence on various sectors from technology to finance. The next time you see dust particles dancing in a ray of sunlight, take a moment to appreciate the unseen forces at work, orchestrating the dance of Brownian motion.