Table of Contents
Alpha Particles
Alpha particles are helium-4 nuclei, symbolized as $He_{2}^{4}$, consisting of two protons and two neutrons. They have a mass of approximately 6.6464835 x 10⁻²⁷ kg (4u – atomic mass units, 4 X the mass of a proton) and carry a positive charge of +2e. Due to their positive charge, alpha particles are deflected by electric and magnetic fields. Their ionizing power is high, making them capable of removing electrons from nearby atoms or molecules very effectively and producing a large number of ions along their path.
The range of alpha particles in air is about 3-4 cm, and they can be easily stopped by a piece of paper. They cause substances like zinc sulphide to fluoresce and can blacken photographic plates. Alpha decay can be represented by the equation:
$$P_{Z}^{A} \rightarrow D_{Z – 2}^{A – 4} + He_{2}^{4}$$
where $P$ is the parent nuclide and $D$ is the daughter nuclide. Alpha particles travel at speeds up to about one-tenth of the speed of light and have a significant impact on ionization due to their mass and charge.
Beta Particles
Beta particles are high-energy electrons, with energies of a few MeV, originating from the nucleus during a nuclear transformation where a neutron converts into a proton and an electron. These particles can reach speeds up to 50% the speed of light and are easily deflected by magnetic and electric fields. The ionizing power of beta particles is about 1/10th that of alpha particles, but they have a greater range in air, able to travel several meters and are stopped only by a few millimeters of aluminum.
The general equation for beta decay is:
$$P_{Z}^{A} \rightarrow D_{Z + 1}^{A} + e_{-1}^{0}$$
Beta particles, being electrons, carry a negative charge of -e and have a mass $\frac{1}{1836}$ times that of a proton. They can cause certain materials to fluoresce and also blacken photographic plates.
Gamma Rays
Gamma rays are electromagnetic waves with wavelengths shorter than X-rays, making them electrically neutral and not deflected by electric and magnetic fields. They possess the strongest penetration power among the three types of radiation, requiring several centimeters of lead to be stopped. Gamma rays have a low ionization power compared to alpha and beta particles. Their emission signifies a nucleus transitioning from a higher to a lower energy state, often accompanying the emission of alpha or beta particles. Gamma rays are massless and represent the energy released during nuclear transitions.
Ionization & Penetrating Power
Ionization refers to the process of forming charged atoms (ions) by losing or gaining electrons. Alpha particles have the strongest ionization ability, knocking electrons out of atoms with ease due to their mass. Beta particles, being lighter, are less effective at ionizing atoms compared to alpha particles but more so than gamma rays, which are the least ionizing due to their uncharged nature.
In terms of penetrating power, alpha particles are the least penetrating, stopped by a sheet of paper, while beta particles can be halted by a thin sheet of aluminum. Gamma rays, on the other hand, exhibit the most significant penetrating ability, with their intensity reduced to half by 1-2 cm thick lead.
Deflection Of Radioactive Particles
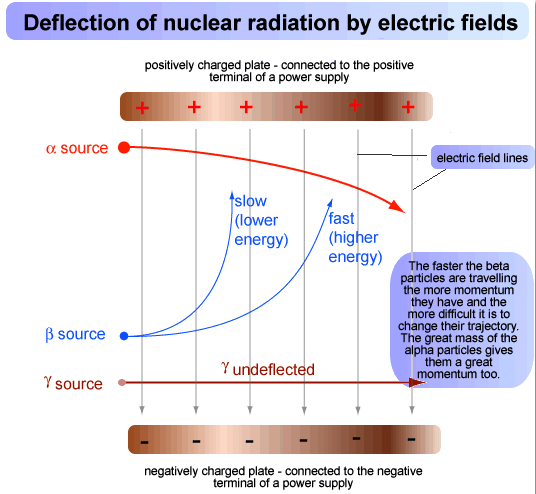
Deflection Of Radioactive Particles In An Electric Field
In the diagram above, a radioactive source is placed at one end of an electric field. The behaviour of the three types of emission in an electric field:
- The α-particles behave like positively charged particles and they are deflected towards negative plate in a parabolic path.
- The β-particles behave like negatively charged particles and are deflected towards positively charged plate. β-particles show much greater deflections than α-particles because of their lower mass.
- The γ-rays show no deflection in the electric field and travel in a straight line as they have no charge.
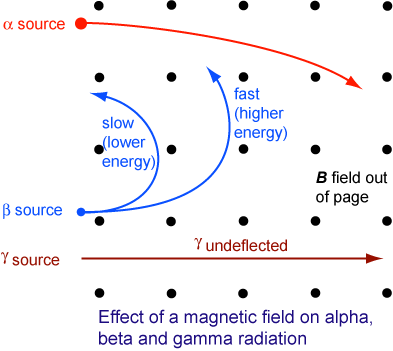
Deflection Of Radioactive Particles In A Magnetic Field
The figure above illustrates the behaviour of the three types of emission in a magnetic field. (The magnetic field is coming out of the page as denoted by the dots.)
- The α-particles and β- particles are deflected in directions given by Fleming’s left hand rule.
- α-particles are positively charged. The direction of movement of α-particles can be considered as in the direction of conventional current flow (i.e. left to right). Therefore, the force on them must be towards the bottom.
- β-particles are negatively charged. The conventional current flow is in the opposite direction to the movement of β-particles (i.e. right to left). Therefore, the force on them must be towards the top.
- γ-rays are not affected by the magnetic field and they travel in a straight line.
Summary Table
The following table summarizes the characteristics of alpha, beta, and gamma rays:
| Property | Alpha (α) Rays | Beta (β) Rays | Gamma (γ) Rays |
|---|---|---|---|
| Nature of Radiation | Helium Nucleus (He²⁺) | Electron (e⁻) | Electromagnetic radiation |
| Ionizing Power | High | Low | Very low |
| Penetrating Power | Very low (Stopped by paper) | Low (Stopped by aluminum) | Very high (Reduced by lead) |
| Effect of Magnetic Field | Deflected (large radius) | Deflected (small radius) | No effect |
| Effect of Electric Field | Attracted to negative (-) | Attracted to positive (+) | No effect |
Worked Examples
Example 1: Alpha Particle Deflection
A radioactive source emits alpha particles towards a negatively charged plate. Describe the path of these alpha particles and explain why this path occurs.
Click here to show/hide answer
Alpha particles, being positively charged ($He_{2}^{4}$ nuclei with a +2e charge), will be attracted towards the negatively charged plate. The path taken by these particles will be parabolic, curving towards the plate. This curvature occurs due to the electric force acting on the positively charged alpha particles, pulling them towards the opposite charge (negative plate) in accordance with Coulomb’s law.
Example 2: Beta Particle Penetration
A beta particle (a high-energy electron) is emitted from a radioactive source and travels through air. Explain how far it might travel and what material could stop it.
Click here to show/hide answer
Beta particles can travel several meters in air due to their relatively small mass and high speed (up to 50% the speed of light). However, they can be stopped by a few millimeters of aluminum. This is because, as the beta particles pass through the aluminum, interactions with the atomic nuclei and electrons in the material absorb the beta particles’ energy, effectively halting their travel.
Example 3: Gamma Ray Emission
Explain why gamma rays require several centimeters of lead to stop them, considering their nature and interaction with matter.
Click here to show/hide answer
Gamma rays are electromagnetic waves with extremely short wavelengths, making them highly penetrating. Unlike alpha or beta particles, gamma rays carry no charge and have no mass, allowing them to pass through most materials with ease. Lead is dense with tightly packed nuclei, providing a high probability of interaction between gamma rays and the lead nuclei. This interaction results in the absorption of the gamma rays’ energy, but due to their high penetration power, several centimeters of lead are required to significantly reduce their intensity.
Example 4: Ionization by Alpha Particles
Describe the process and outcome when an alpha particle ionizes an atom in its path.
Click here to show/hide answer
As an alpha particle (consisting of two protons and two neutrons) travels, its positive charge can attract electrons from nearby atoms. When an alpha particle comes close enough to an atom, its electric field can pull away one or more electrons from that atom, ionizing it. This process results in the formation of a positively charged ion (the atom that lost electrons) and free electrons. The ionization process by alpha particles is highly efficient due to their significant mass and charge, leading to the production of a large number of ions along their path.
Example 5: Deflection in a Magnetic Field
A beam containing alpha particles and beta particles is passed through a magnetic field perpendicular to their motion. Describe the direction of deflection for each type of particle.
Click here to show/hide answer
In a magnetic field, the direction of deflection for charged particles can be predicted using Fleming’s left-hand rule. For alpha particles, which are positively charged, the direction of conventional current is the same as their direction of motion. Applying Fleming’s left-hand rule, if the magnetic field is coming out of the page, alpha particles will be deflected downwards.
Beta particles, being negatively charged, move in the opposite direction to the conventional current. Therefore, applying the same rule but considering the opposite direction for current flow, beta particles will be deflected upwards in the magnetic field.
These deflections occur because of the Lorentz force acting on moving charges in a magnetic field, which is perpendicular to both the direction of motion and the magnetic field, causing the particles to follow curved paths.